Tidepool Loop App for Automating Insulin Dosing Now with FDA
UPDATE: Tidepool received FDA clearance on Jan. 23, 2023
Tidepool Loop, a first-of-its-kind app that will connect with a variety of compatible insulin pumps and continuous glucose monitors (CGMs) to automate insulin dosing, has been submitted for FDA approval.
This is big, Diabetes Friends, because new automated insulin delivery (AID) systems are the future of diabetes care, and because the Tidepool Loop project represents over two years of development work aimed at bringing do-it-yourself (DIY) "artificial pancreas" innovation into an official, FDA-regulated product that will be broadly available via the iOS app store.
To date, the diabetes startup has focused on building a cloud-based platform that lets people collect and jointly review data from different glucose meters, insulin pumps, and CGMs. As of early 2021, that entails over 50 different devices along with the ability to upload and interact with that data.
The new Tidepool Loop innovation continues the mission, but takes it to the next level, says Tidepool founder Howard Look, whose inspiration is his daughter, who was diagnosed with type 1 diabetes (T1D) in 2011.

"The vision is about creating an ecosystem, where you get to choose what's right for you as a person with diabetes," Look tells DiabetesMine.
"This doesn't get us all the way there, but it's a big step in the right direction. This pushes everyone forward, to thinking about interoperability and interchangeability, and that makes for a better world for people with diabetes."
Why is Tidepool Loop a big deal?
Once referred to as "artificial pancreas" technology, new AID systems — also known as closed looping — essentially mimic what a healthy pancreas does: monitor glucose levels and deliver insulin as needed. It takes a lot of the guesswork out of managing diabetes and helps keep the user within a healthy blood glucose range 24/7.
Medtronic and Tandem Diabetes Care currently have their own systems available, and more developers are building their own AIDs to launch in the coming years.
But even before any commercial system was available, people with diabetes (PWDs) began creating their own homemade DIY artificial pancreas systems. DiabetesMine has been involved in encouraging this effort, rallied around the grassroots #WeAreNotWaiting movement.
Thousands now use homemade systems called OpenAPS, AndroidAPS and Loop worldwide, but the downsides have held many people back: the complex build-it-yourself process; having to rely on older, non-warranty devices; safety fears; and the lack of an established tech support infrastructure beyond the open-source community.
That's all about to change, thanks to open-data nonprofit Tidepool.
Tidepool is building a first-ever commercial version of Loop that would do away with all of those downsides, while offering unprecedented ease of use via smartphone control.
For those who use the original open-source Loop system, don't fret. That isn't going away. The DIY versions of Loop will still exist and continue to evolve even as this new Tidepool Loop option materializes.
But this marks the first time a crowdsourced DIY diabetes solution will morph into an "official" FDA-approved product, that can compete with offerings from established vendors.
This move essentially takes DIY tech that's been largely a "use at your own risk" proposition into the mainstream, allowing for a product that's not only FDA cleared but also one that healthcare professionals may be more comfortable prescribing and discussing with their patients.
Also, users would now be able to easily use the Tidepool Loop app with any supporting insulin pump or CGM device they may choose — no more scrounging for outdated, used Medtronic models (still the only option for those who don't use the Omnipod).
The hope is to gain FDA approval ASAP in 2021, to be able to launch the mobile app by year's end.
A quick guide to #WeAreNotWaiting diabetes terms
Since there's quite a bit of insider jargon here, let's begin with a refresher (primer?) on the #WeAreNotWaiting movement and the key components involved:
#WeAreNotWaiting: Hashtag #WeAreNotWaiting is the rally cry of folks in the diabetes community who are taking matters into their own hands by developing platforms, apps, and cloud-based solutions, and reverse-engineering existing products when needed in order to help people with diabetes better use devices and health data for improved outcomes. The idea is, "We are not sitting back and waiting for the authorities to make these things happen for us." Note that the term was actually coined at our first-ever DiabetesMine D-Data ExChange gathering at Stanford University in 2013.
The DIY movement began most notably with Nightscout, a tool for remote data-sharing via mobile app, website, and smartwatch. That was before any manufacturers launched their own products with these capabilities. Of course, interest in digital health tools has exploded over the past several years, with burgeoning closed-loop functionality at the center of that in the diabetes world.
iCGM: A regulatory designation the FDA established in March 2018, to indicate CGMs that are designed to be interoperable with other mHealth devices. To date, only the Dexcom G6 has this designation, but it sets up a framework for future connective CGM devices to be approved via a simpler regulatory process. The end game is making it easier for PWDs to choose between the various devices we want to use, knowing they'll be able to "talk to each other."
ACE pump: An alternate controller enabled (ACE) pump is the FDA designation created in February 2019 for interoperable insulin pumps. Currently, the Tandem t:slim X2 is the only insulin pump with that label, but other manufacturers are working toward securing it for their future products.
RileyLink: D-Dad Pete Schwamb from Minnesota is the inventor behind this little box that's become a critical part of many DIY closed loop systems. It handles communication between the insulin pump (those older models) and the CGM. It's basically the radio bridge between the devices — speaking the Bluetooth LE language of the iPhone and converting it to 900Mhz frequency that the older Medtronic pumps use. That allows for communication with mobile apps, like Loop, used to control the insulin-dosing functionality via a smartphone or mobile watch.
Loop: A DIY automated insulin delivery algorithm app that operates as the "brain" of this particular homemade closed-loop system, containing the algorithm and user interface to control the insulin-dosing and AP functionality. As noted, to date this solution has been completely open-source and non-commercial. It's estimated that 10,000 to 15,000 people globally are using Loop. An open-source website called LoopDocs contains the community-created and maintained documentation and instructions for building a DIY Loop system.
OpenAPS: Another DIY closed-loop system initially developed by Dana Lewis and Scott Leibrand that's one of the most visible examples of this homemade technology. Lewis, who lives with T1D, started creating it in 2013 and it's evolved with community input ever since. It also uses an algorithm to control the insulin dosing functionality, though unlike Loop where the algorithm is based on the phone app, OpenAPS uses a separate Edison mini-computer or related item to act as the brains of the system.
AndroidAPS: Stemming from Europe, this is a Droid-based version of the OpenAPS mentioned above. It works largely the same way, and its development has been mainly led by D-Dad Milos Kozak, a software developer from Prague, Czech Republic.
FDA Digital Health Software PreCertification Program: In 2017, the federal agency launched a pilot program designed to speed up the regulatory process for health software by creating a "trusted network" system of developers. Tidepool was one of nine companies chosen to participate, and they're now part of regular meetings to determine the best protocols that can be used when making new apps or software. As part of this FDA pilot, Tidepool is working with regulators — and their new Tidepool Loop project is viewed as a potential "test case" that can be used for future DIY technology that may be brought into the regulated commercial space.
JDRF Open Protocols Initiative: Launched in late 2017, the JDRF Open Protocols Initiative aims to encourage device manufacturers to build products that are interoperability-ready. JDRF's goal was really to set a baseline for "plug-and-play" diabetes technology, for a world in which PWDs can pick and choose the particular devices they might want to use, and know that they will function well together. While Tidepool Loop isn't directly tied to this initiative, the efforts certainly overlap and the goals go hand-in-hand.
Overcoming hurdles, cracking open interoperability

Tidepool has long described the goal of Tidepool Loop as helping the D-community "overcome several challenges that prevent widespread adoption of these incredible projects." In the company's words:
- For most people, their only option is to buy an old, used Medtronic pump. We think that’s just not right. People should be able to use officially supported and commercially available pumps. We shouldn't have to buy old, unsupported, out-of-warranty pumps on Craigslist, eBay, or Medwow to get great care.
- Not everyone is comfortable building and maintaining their own DIY system.
- Many people with diabetes are not comfortable using a system that isn’t FDA cleared or approved. And many doctors and Diabetes Care and Education Specialists (DCES) are not willing to recommend a product to their patients that is not FDA cleared or approved. The FDA would really love for there to be an entity that takes responsibility for support and tracking of safety and efficacy, including “post-market surveillance” (the fancy term for "gathering and analyzing data to make sure a pharmaceutical drug or medical device is
safe and effective after it ships").
Tidepool is tackling those barriers head-on.
Q&A on the details of Tidepool Loop
DiabetesMine spoke with the Tidepool team, including Look, following their FDA filing on Dec. 17, 2020, to get the scoop on this new app, the backstory, and how it will be supported.
How would you describe this 'iController' app?
Think of it like an equation: a compatible pump + a compatible CGM + controlling algorithm = a closed-loop system. While some systems currently available (like the Medtronic, Tandem, and upcoming Omnipod technologies) weave those smart algorithms into their systems, this new Tidepool Loop app will allow for a separate piece to work with the pump and CGM.
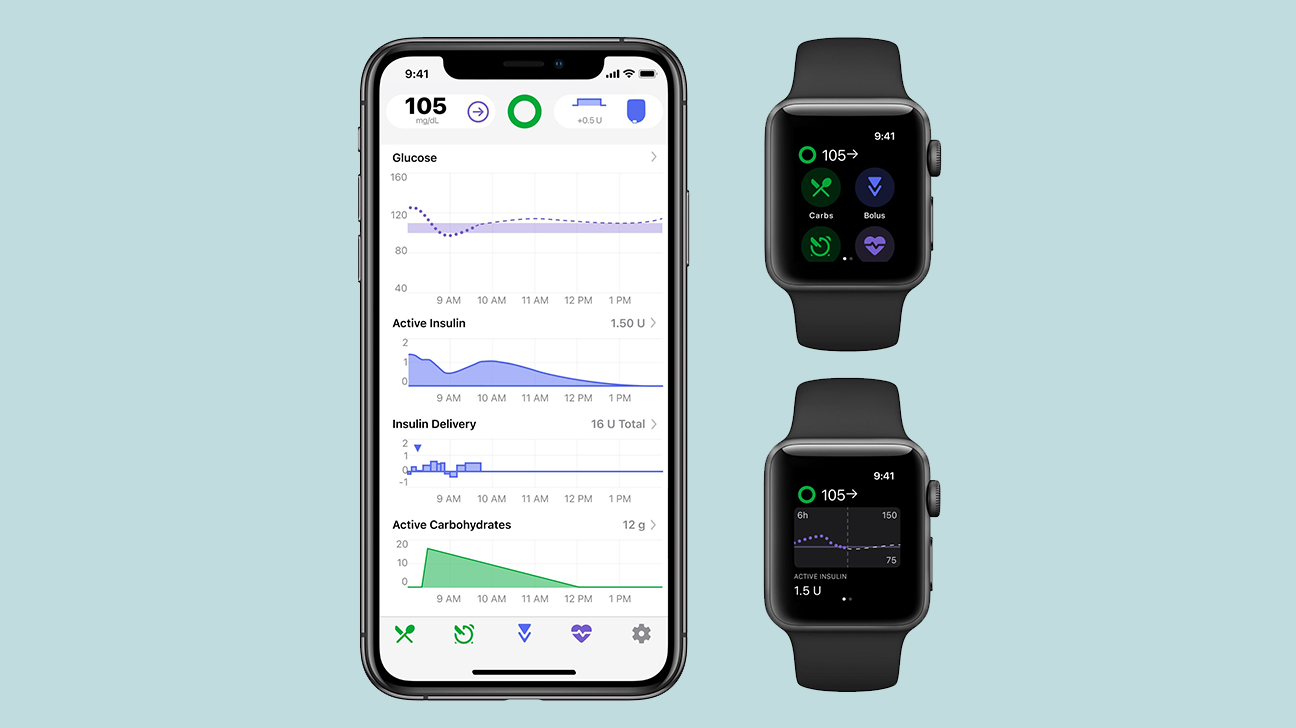
"What if instead of going to one or two companies for each piece of the system, you had a third party with an app allowing for that interoperability?" explained Melissa Lee, a longtime T1D advocate who is Tidepool's marketing and clinical training manager. "Those three pieces make up the system, and it's the (algorithm) piece that we're doing."
The goal is to modify basal rates every 5 minutes. The algorithm will look at your insulin on board, current glucose reading, and entered carbs that you plan to eat, and adjust the basal rates to reduce or avoid high and low blood sugars.
Will this offer customizable glucose targets?
Tidepool has asked the FDA for adjustable glucose targets, something the agency hasn't before allowed. Compared to existing closed loop tech with fixed targets — Medtronic's 670G at 120 mg/dL and Tandem's Control IQ at 112 mg/dL — the Tidepool app submission proposes personalization for those settings. It's TBD whether the agency will OK that idea for Tidepool Loop's mobile app.
How exactly does it differ from the DIY Loop version?
While Tidepool needs to wait for FDA clearance before discussing specific features, they remind the D-Community about data from the JAEB Loop Observational Study of DIY Loop to help support their submission.
Any changes that Tidepool has made to the DIY product have been made in a way that they believe will be supported by the study data and Tidepool’s own usability testing data. The company says they look forward to publishing full submission documentation once the agency has had a chance to complete their review.
Look says they also plan to keep the "DIY Loop experience" that many in the #WeAreNotWaiting community are familiar with, and that may include pizza-specific boluses that can be extended for longer-impacting meals like that. Fun features like including emojis may also remain a part of Tidepool Loop!
Which phones will it be compatible with?
At launch, it will work on iPhone and iPod Touch. Eventually, Android compatibility will be added as well.
What about connecting with smart insulin pens?
Look says: "That's a very interesting path. We have nothing to announce, but I'm a big believer in connected pens and it could be something we think about in the future."
How much will Tidepool Loop cost?
"We would really like Tidepool Loop to be as accessible, broadly and fairly and equitably, as possible. There are a lot of ways we can make that happen and we're exploring that, especially as we are a nonprofit," Look says.
To date, everything Tidepool has offered (prior to Tidepool Loop) has been made available for no cost to the end-user. That's been possible through corporate sponsorships, JDRF and Helmsley Charitable Trust grants, as well as D-Community donations.
"We are keenly aware that asking end users to pay for Tidepool Loop out of pocket would be a bad idea," Look said. "We'll keep the community updated as we work through device accessibility and insurance. It's too early to say at this point on how that will all go for potential users."
Will the company offer tech support for Tidepool Loop users having issues with their CGM or pump?
Look says: "I think it's fair to say we won't get into the hardware distribution business. We aren't going to be a single point of contact to get supplies or devices. To the extent that we can collaborate with our device partners to make it easier for people to get bundles, that is a great opportunity that we can look at."
How will you approach your own customer service for this app?
Tidepool's goal is to provide delightful and empathic support for our users, and this is where us having "pancreas in the game" matters. They understand what it's like to live with diabetes, and how important having good customer support is.
"With Tidepool Loop, we'll have a wonderful opportunity to innovate on this because (users) will be holding the product — a mobile app — on their phone in their hand. So that allows us to provide some of the support on the phone directly, and we're exploring all of that," Look says.
Who are Tidepool's device partners?
Tidepool says it has a "dance card" that continues to evolve. As of early 2021, partners include Insulet with its Omnipod tubeless pump, Medtronic, and Dexcom. They will also likely work with the Tandem t:slim X2 down the road, given that device has an ACE/iPump designation. In the past, Tidepool has told DiabetesMine that they'd likely work with other pump and CGM makers involved in the JDRF Open Protocols Initiative: Roche, Sooil, SFC Fluidics, and Ypsomed.
How impactful was the JDRF Open Protocols effort on getting you to this point?
Tidepool says JDRF did the industry a world of good in establishing the Open Protocols Initiative.
Coupled with the consistent advocacy from both JDRF and the Helmsley Charitable Trust to bring together device makers, regulators, and legal experts, the initiative gave credibility to the concept of device interoperability. Now the work lies with these many stakeholders to work out the details from a business and regulatory perspective, "but we couldn’t be pursuing it if the initiative hadn’t generated the momentum," Look says.
How does Tidepool view the competitive 'race' to get closed loop technology to market?
Look says: "We see it more as pieces of a puzzle coming together. We believe a rising tide lifts all boats. If our submission can help create pathways of innovation for new algorithm creators or existing large medical device companies, it’s the people with diabetes and their care teams who ultimately benefit."

Tidepool was hit hard by the pandemic and needed layoffs. How does that impact your work now?
"Practically, we've always been a remote organization and gave up our small San Francisco office in 2017. We've been completely virtual before it was necessary. So from that perspective, that switch wasn't a big impact on us. But the financial impact has been tough," Look says.
"The pandemic hit a lot of nonprofits hard, including us. We had to reduce expenses, and that meant doing one of the saddest things we've ever done: saying goodbye to some incredibly talented and capable people, in order to get through this dark period. But we were able to hunker down and continue to execute on our mission."
Look continued, "It's been hard, and I won't pretend I ever want to go through that again, but I'm thankful for every single person on board who helped get us to this point, as well as the people who are still on the team helping us continue on with our mission. I'm optimistic that the pandemic will end, fundraising will get back to normal, and then we'll be able to move forward."
How quickly do you expect Tidepool Loop to move through the FDA review process?
Being one of the first companies in FDA’s software pre-certification pilot program affords the company more frequent communication with FDA. They tell DiabetesMine that the FDA has been extraordinary to work with so far, and they don’t expect that to change.
"What we can’t account for is the delay in review cycles that we’re seeing across industry due to the urgent focus for FDA that is COVID-19," Look says.
- - - - - - - - - - - - - - - - - -
Originally published on DiabetesMine on Jan. 13, 2021

Comments